Questionable COVID Results - More Concerning Whistleblower Allegations From State COVID Lab
(CBS13) — Several new whistleblowers have come forward following a CBS13 investigation that revealed concerning allegations from inside California's billion-dollar COVID testing lab.
The new whistleblowers reached out to confirm the previous reports and to share new allegations including concerns over compromised COVID results.
More Whistleblowers, More Allegations
What began with one whistleblower has evolved into a steady stream of current and former CDPH-PerkinElmer lab employees who want the public to know what they say is happening inside the state's new COVID-testing lab.
The whistleblower who was featured in our original report wasn't the first to reach out to us with concerns. However, "Dottie" was the first to agree to an on-camera interview. She confirmed the allegations and evidence provided by others.
Since "Dottie" came forward, we've heard from several more whistleblowers, ranging from lab assistants to management, who wanted to share their stories too.
"I was just relieved I wasn't the only one feeling this way," said a whistleblower we'll call Nicole. She said she learned about our reports from a company-wide PerkinElmer email and felt compelled to reach out to verify the allegations and share other concerns.
Another whistleblower we'll call "Mary" said she wasn't at all surprised by our initial reports and "Beth" said she came forward because "I still think that there are more shocking things that are going on."
Like Dottie, most of the new whistleblowers asked that we conceal their identities.
ALSO READ: Asleep At Lab: Whistleblower Allegations From Inside CA's Billion-Dollar COVID Lab
"I don't want to face any backlash," Nicole explained. "They're paying everybody really well and I'm worried that some people (are) going to be really mad at the whistleblowers and maybe retaliate."
"I just don't want to get blacklisted," Mary added, noting that the clinical laboratory community in Southern California, where the lab is located, is relatively small.
However, after seeing our reports, former Laboratory Manager Mahnaz Salem, PhD reached out and offered to speak on the record.
"I really want (the) public to know that this lab should not continue operating like this," explained Dr. Salem, who recently resigned from the lab. She was previously a state laboratory inspector.
Each of the allegations that we've publicly reported were corroborated by multiple current and former employees.
ALSO READ: CBS13 Investigation Reveals Thousands of Inconclusive COVID Results From State Lab
Confirming Initial Reports
The new whistleblowers confirmed the allegations from whistleblowers in our previous reports. Several said that it was not uncommon to see lab techs watching videos or sleeping while processing nasal swabs for testing. One whistleblower provided a new photo of someone sleeping the week our story aired.
The new whistleblowers also verified the validity of the Quality Control reports and the emails we obtained that described incidents of contamination, swapped samples, and testing errors between November and late January.
"Samples would go missing or they would be switched," Nicole said.
Every whistleblower we've heard from also confirmed instances of unsupervised staff processing patient samples before completing the required training or getting signed-off for competency, which is a violation of laboratory regulations.
"It is true that unlicensed, unsigned off people were operating instruments," said Beth.
"The staff are very untrained and unsupervised," Mary said. "Some people don't even know how to pipette, which is a critical skill."
"(The lab) is still just as disorganized as it was three months ago when I first started," Nicole added. "Of all of my past experiences in other clinical labs, I've never witnessed anything like this."
CONTINUING COVERAGE: CBS13 Investigates Problems at California's COVID Testing Lab
Complaints Ignored
The whistleblowers say they're coming to us after their repeated complaints to management were ignored.
"I complained to management and I got zero emails back," Mary said.
"I've never received an email back from HR," Nicole added.
"It feels like nobody listens to anybody in the lab, Beth said.
"That was part of the culture, they would just not respond," Mary added.
CBS13 has reviewed several emails from whistleblowers sent to HR, for which they say they received no response. Every whistleblower we heard from cited complaints to HR or management that, they say, were ignored.
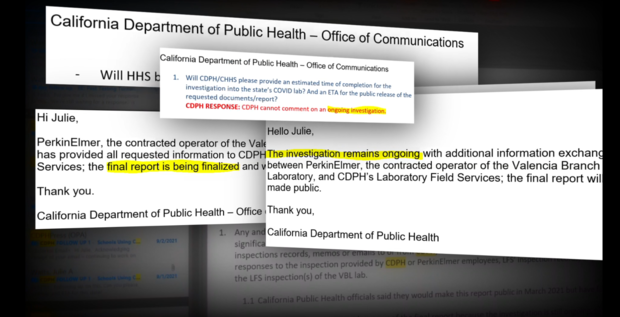
In response to these allegations, CDPH said in an email:
"PerkinElmer and CPDH know that lab personnel are the first line to ensure the high performance and quality of the lab operations, and processes and channels are in place to ensure personnel have ample opportunity to report any and all concerns. Without any information about the "various issues" or "verbal concerns" we cannot comment on the topics purportedly raised."
No Process to Immediately Fix Wrong Results
The whistleblowers acknowledge that mistakes happen in all labs. However, they stress that there is supposed to be a procedure to immediately correct them.
At the CDPH-PerkinElmer lab, they say, that wasn't always the case.
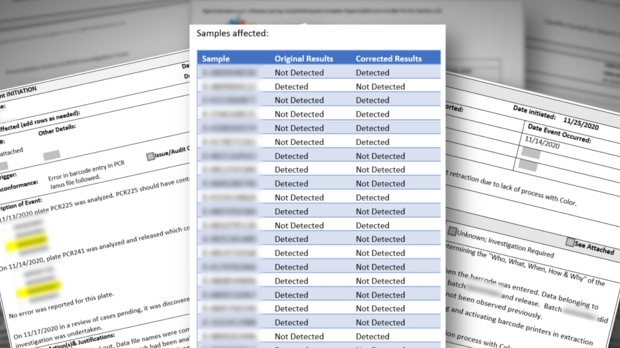
For instance, several whistleblowers pointed us to incidents where wrong COVID results were released. Quality Control (QC) reports indicate that, for weeks, there was no process in place to immediately notify people that they had received the wrong test results.
"They released those results but they didn't have a mechanism in place to issue (a) corrected report immediately," Dr. Salem explained.
For example, according to one Quality Control report, and subsequent corrective action documents, 22 wrong results were released due to a barcode entry error on November 14, 2020.
According to the documents, the mistake was discovered three days later, "but no process for correcting reports was in place."
Whistleblowers explained that meant people who were actually positive received negative results and vice versa, but there was no way to immediately notify them about the wrong result.
They point to the ripple effect of false-positive results that have the potential to shut down businesses and schools. Worse, they worry, that people who were positive, but thought they were negative, could have exposed vulnerable loved ones over the holidays.
Records indicate seven days after the wrong results were released, the lab finally contacted Color, the company that manages the software used to report results.
But there was still no process in place to immediately correct wrong results.
A corrective action investigation set a December 4, 2020 "Due Date" to establish "the process for requesting a report to be retracted." That was 20 days after the wrong results were released.
"I mean, there's no excuse (for the delay). It's tragic," Mary said.
"Not only that, but it repeatedly happened," Dr. Salem alleged, pointing to subsequent QC reports.
CBS13 sent copies of the documents to CDPH to review.
In response, CDPH said:
"This demonstrates that the quality improvement process worked. An issue was identified during the initial days, it subsequently was documented and resolved. More importantly, new procedures were developed and instituted to prevent it from happening again. As has been noted before, this is an iterative process, and the laboratory is continuously improving."
We shared the CDPH response with whistleblowers ahead of airing this report. They clarified that, despite the December due date, the lab did not actually have a process in place to correct wrong results until this month.
Shortly before this story aired, they provided us with documentation outlining the process for amended and corrected results. It indicates the process was approved and became effective on February 8th, 2021, which was months after the "due date" and the first known instance of swapped results.
CDPH did not immediately respond to our request for an updated response.
CONTINUING COVERAGE: CBS13 Investigates Problems at California's COVID Testing Lab
Concerns Over Compromised Test Results
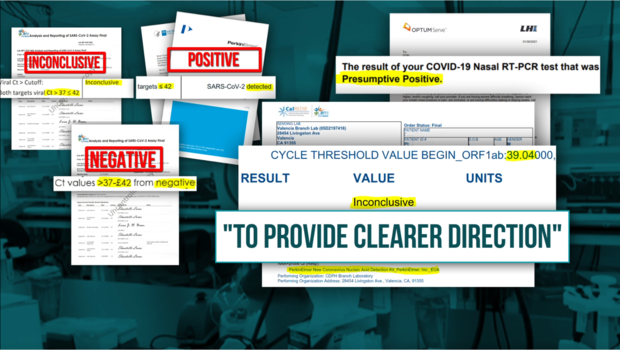
Whistleblowers also provided evidence that, in some cases, COVID test results were released despite quality control failures during a test run.
This was one of their biggest concerns. As Mary explained, when the quality control fails, "you can't use those results."
"This is absolutely against any laboratory procedure," Dr. Salem added.
CBS13 reviewed an email chain from the end of January in which an employee tells managers that she and her team "don't feel comfortable releasing results with (a quality control) failure without written documentation from the Sign off Manager."
The employee said that she had done so "per (a manager's) request."
The email chain also indicates that the employee was reprimanded by management for sharing her concerns in a group email.
A manager appears to say that sending a mass email, "doesn't look good for the company." The last three letters of the word "company" were cut off in the photo of the email that was reviewed by CBS13.
We shared the photo with CDPH, noting that it indicates a manager instructed an employee to release results despite a QC failure. We clarified that multiple whistleblowers allege – and the email indicates – that this was not an isolated incident.
We also noted that the employee appears to have been reprimanded for sharing her concerns with the team.
In their response, CDPH did not specifically address the potentially compromised results or the alleged practice of releasing results despite quality control failures.
"The image you sent of the email is only a part of an extended email chain in which lab personnel emphasize the need to maintain active, complete and direct communication and to ensure proper documentation of all deviations from the standard operating practices. Furthermore, the comment you reference refers specifically to an employee including an entire department in a discussion only relevant to a manager and the employee. In fact, the full thread shows that the management engaged in a discussion with employees to ensure compliance and understanding of laboratory processes."
CBS13 reviewed a copy of the lab's Standard Operating Procedures (SOP) for "Analysis and Reporting," effective as of January 25, 2021. It detailed five major revisions since December 8, 2020.
The document indicates, and whistleblowers allege, that it was not until January 25th that the lab updated its procedures to require that failed positive controls should always trigger a repeat test.
CBS13 sent a copy of the document to CDPH to review. The agency did not immediately respond.
Rushed Results
Whistleblowers say, part of the problem, is a push to return results quickly. PerkinElmer's $1.7 billion contract with the state requires the lab return results in 24-48 hours by March.
"We were under pressure to do a certain number of runs every night," Mary said. "Speed definitely compromised (some) tests."
Nicole agreed that she feels there is a focus on "quantity over quality."
In response to the concerns, CDPH said:
"Timely processing of samples is a critical component of the state's COVID-19 testing strategy. Timely turn-around times mean getting cases isolated faster, and thereby, reducing the spread."
Though, whistleblowers stress that timely results are only effective if they are accurate and clearly identify positive cases. This lab releases a high number of "inconclusive" results.
David vs. Goliath
For the whistleblowers, they say this is a David versus Goliath story. They point out that the California Department of Public Health (CDPH) is now investigating its own lab.
"It's a conflict of interest," Mary stressed.
The whistleblowers would like to see an independent investigation by an impartial body, like the California State Auditor or the Center For Medicare & Medicaid Services (CMS), which is the federal laboratory regulator.
Whistleblowers hope that publicizing the problems will force regulators to take action.
"I'm hoping that with these stories coming out, their fear of having bad PR will actually motivate them to change," Nicole said. She believes that "before the story, they did not care."
"Every patient sample deals with somebody's life," Beth added.
"I think the public deserves to know what's going on in their state lab," Mary stressed, "and everybody deserves to have correct results."
In response to the concerns over a conflict of interest, CDPH paid:
"Laboratory Field Services is the state entity responsible for licensing and investigating laboratories in California. It is not uncommon for them to also license and investigate state-run laboratories. That said, at the request of the state, PerkinElmer is going through a third-party accreditation process by the College of American Pathologists or CAP."
NOTE: This story was updated to include additional information about Quality Control failures that were identified in the SOP reviewed for this Feb 19 report.
WATCH THE CBS NEWS SACRAMENTO SPECIAL REPORT
THE COVID LAB: State Secrets Exposed
This 30-Minute Special Report is the culmination of 14 months of reporting that prompted state and federal investigations, resulted in two new pieces of legislation, and shined a spotlight on shocking public health failures that it appeared regulators tried to hide.
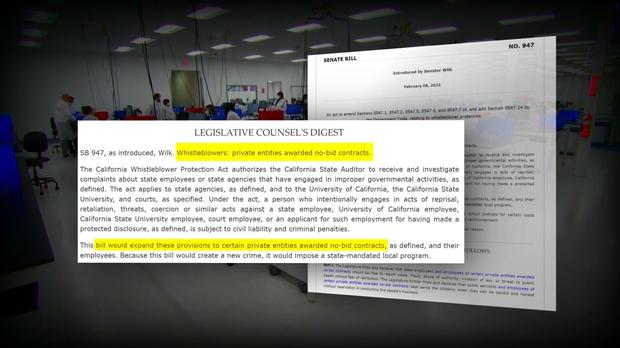
Following these reports, lawmakers introduced legislation that is intended to protect whistleblowers and ensure accountability and transparency long after the pandemic is over. The state ultimately terminated its $1.7B COVID contract with PerkinElmer.