Regulators Find 'Significant Deficiencies' At CA COVID Testing Lab - Whistleblowers Respond
SACRAMENTO (CBS13) — The California Health and Human Services Agency (CHHS) announced Monday that they found "significant deficiencies" at the state's CDPH-PerkinElmer COVID testing lab.
This is the same lab that has been the focus of a CBS13 whistleblower investigation, but lawmakers were told Sunday night that these findings are separate from the state's ongoing investigation into those new whistleblower allegations.
"Significant Deficiencies"
In a press release, the state cited significant deficiencies found during an inspection of the California Department of Public Health (CDPH)-Perkin Elmer lab back in December. State regulators refer to the lab as the Valencia Branch Laboratory (VBL).
CONTINUING COVERAGE: CBS13 Investigates Problems at California's COVID Testing Lab
HHS said, "The Laboratory Field Services Division of the California Department of Public Health (CDPH), which regulates laboratories in the state, found significant deficiencies... during an initial routine inspection that occurred in early December when the laboratory first opened."
The lab began processing Californian's COVID tests on Nov 2.
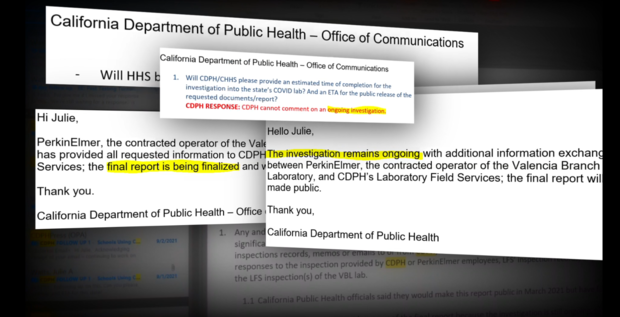
The press release added, "In the interest of transparency, they've agreed to make this public now instead of waiting until the full report is finalized mid-March. However, they did not reveal what the "significant deficiencies" were.
The state said inspectors were "confident these deficiencies will be quickly remedied to avoid any impact on the laboratory's license."
In a separate press release, PerkinElmer said "the deficiencies identified by LFS have long since been resolved."
ALSO READ: Questionable COVID Results - More Concerning Whistleblower Allegations From State COVID Lab
Whistleblowers Respond
Several of the whistleblowers told CBS13 they were concerned by the state's implication that any lab deficiencies date back to December and have since been addressed.
CBS13 has heard from a steady stream of whistleblowers who share ongoing concerns that they say were ignored by lab management.
"It is still just as disorganized as it was three months ago when I first started," insisted a whistleblower we're calling "Nicole."
Their new allegations range from unlicensed lab techs sleeping on the job to incidents of contamination, swapped samples, and incorrect results released without a procedure to immediately notify people that they received the wrong results.
Whistleblowers also said unsupervised staff have been processing patient samples before completing training or getting signed-off for competency, which is required by law.
ALSO READ: Asleep At the Lab: Whistleblower Allegations From Inside CA Billion-Dollar COVID Lab
"The staff are very untrained and unsupervised," said "Mary," another whistleblower.
Competency Record Concerns
CDPH told CBS13, and later lawmakers, that, "[i]t's the State's understanding that all individuals who are working in the laboratory and are handling specimens are credentialed and trained."
"PerkinElmer Valencia is not being forthcoming with the training status of its workers," "Mary" said Monday in response to the CDPH response.
CBS13 obtained a "competency tracker" from an internal audit that indicated that numerous employees who were processing patient samples had not been signed off for competency, which is required by law.
CBS13 asked CDPH for a response on Super Bowl Sunday.
Within hours, internal emails obtained by CBS13 indicate that managers were called in to address the competency issues during the Super Bowl, "[s]ince this became even more urgent."
The email chain confirms that at least one manager was "headed in to help coordinate" citing a list of dozens of people in the RNA extraction lab alone who did not have competency as "a good place to start."
The competency tracker spreadsheet, which managers referred to in the email chain, contained the names of hundreds of employees who had not been signed off in one or more areas.
Twenty two minutes after the internal email was sent, CDPH responded to CBS13's questions stating, "No employee without sign-off competency is running samples. Once competency was achieved all appropriate paperwork was placed in MediaLab to await Director sign-off."
READ MORE: Questionable COVID Results: More Concerning Whistleblower Allegations From State Lab
Multiple whistleblowers dispute that claim.
"It is true that unlicensed, unsigned off people were operating instruments," said a whistleblower we will call "Beth".
They provided staff competency and work records that indicate some staff were processing samples before they were signed off for competency.
Multiple whistleblowers say they operated instruments for months before being signed off themselves and cite errors made as a result of a lack of training.
Lab Errors and Wrong Results
The state's press release also claims:
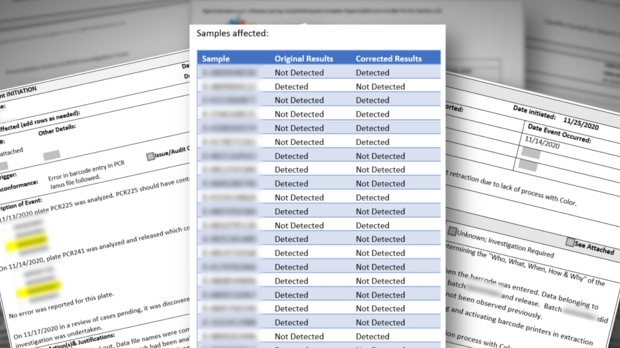
"Out of a total of more than 1.5 million tests performed, the Valencia Branch Laboratory has issued corrected reports for approximately 60 (.0039%) samples and been unable to test approximately 250 samples (.017%) due to lab errors."
Whistleblowers dispute those totals and point to the more than 50,000 results between December and January alone that didn't reveal if the person actually had COVID, including inconclusive, invalid, and lost samples.
"I really want [the] public to know that this lab should not continue operating like this," former lab manager Dr. Mahnaz Salem said.
State Didn't Know Or Denied Knowing
Whistleblowers say they do not think CDPH should be investigating its own state lab.
They point to a conflict of interest and note that, for weeks, the state agency conducting this investigation into its own state lab either didn't know or wouldn't admit that the lab's COVID test was no longer authorized by the FDA.
This is an excerpt from a February 6, 2020 email exchange between CBS13 and CDPH:
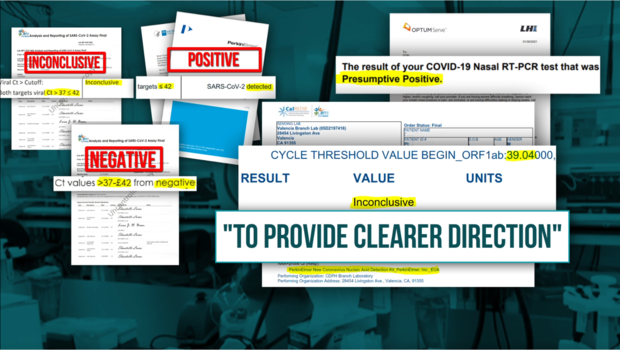
CBS13: When did the lab change their CT range for inconclusive/presumptive positive tests…?
CDPH Response: The Ct range was never adjusted. As has been provided on multiple occasions, effective December 11 changes to the terminology were made to be more directive to the individual receiving the test result. The PerkinElmer EUA allows for a positive up to a Ct value of 42.
CBS13: Is the CDPH/PerkinElmer lab using an (Emergency Use Authorization) EUA Assay or a (Laboratory Developed Test) LDT Assay?
CDPH Response: The laboratory is using the assay approved by the FDA pursuant to the EUA.
CBS13: If EUA, can you provide a copy of the FDA approval for the change to the EUA?
CDPH Response: The laboratory does not need approval from the FDA to change its Ct value cutoffs if within the original EUA parameters. The laboratory is operating within the parameters of the EUA.
Several lab directors we consulted with for this story disagreed with CDPH's assertions. So did several whistleblowers — and the FDA.
It wasn't until CBS13 sent CDPH proof from the FDA that the testing procedures were not authorized, that the state public health agency suddenly changed its response.
CDPH finally acknowledged on Wednesday that instead of the test that was submitted for review and approved under the Emergency Use Authorization (EUA), the lab is now using its own Lab Developed Test (LDT).
The agency claimed it "mistakenly said" it's testing procedures were approved by the FDA.
Whistleblowers question how the California Department Of Public Health could make that kind of "mistake".
While the state's $1.7 billion dollar lab contract requires that the lab use the FDA-approved test, CDPH later pointed to a clause in the contract that allows them to make changes.
However, the contract, which was written on behalf of California taxpayers, also requires that amendments to the contract be "made in writing."
CBS13 asked CDPH for a copy of a signed and dated amendment to the contract that confirms both parties agreed to use a lab-developed test in lieu of the test authorized by the FDA.
The agency has not yet provided a response.
Whistleblowers stress that until the CBS13 report, CDPH led Californians to believe that their crucial COVID tests were still authorized by the FDA.
While lab-developed tests are currently allowed with certain restrictions, they are not reviewed, authorized or approved by the FDA.
"The public deserves to know what's going on in their state lab," a whistleblower said.
In response to the concerns over a conflict of interest, CDPH said:
"Laboratory Field Services is the state entity responsible for licensing and investigating laboratories in California. It is not uncommon for them to also license and investigate state-run laboratories. That said, at the request of the state, PerkinElmer is going through a third-party accreditation process by the College of American Pathologists or CAP."
However, whistleblowers stress that a third-party accreditation process would not necessarily uncover many of the issues they're most concerned about.
Whistleblowers are calling for an independent, forensic investigation of records, policies, and COVID results.
WATCH THE CBS NEWS SACRAMENTO SPECIAL REPORT
THE COVID LAB: State Secrets Exposed
This 30-Minute Special Report is the culmination of 14 months of reporting that prompted state and federal investigations, resulted in two new pieces of legislation, and shined a spotlight on shocking public health failures that it appeared regulators tried to hide.
Following these reports, lawmakers introduced legislation that is intended to protect whistleblowers and ensure accountability and transparency long after the pandemic is over. The state ultimately terminated its $1.7B COVID contract with PerkinElmer.